The company is located in Xinfeng county Ganzhou, Jiangxi province which has concenient transportaion and excellent geographical location and occupies an area of 10,000 square meter,Aclean and standardized factory building of 4,000 square meter that meets the requirements for medical device production is built. acilities, personnesIt is equipped with warehouses and testing experiments, facilities,peronnel disinfection and protection facilities,with 20 automated production lines, can produce of Medical protective coverall, medical surgical gown, medical isolation gown,medical surgical masks,disposable medical masks and daily protective masks,KN95 protective masks,ect.that meet national standards.With an annual output of 200 million of various types ,the company has 100,000-level clean production workshops,10,000-level standardized laboratories and large-scale advanced sterilization equipment. It strictly implements the management style of people flow and logistics diversion, safe and clean production, thorough serilization, advanced automated inspection and testing capabilities ensure that the product quality is safe and reliable and can provide various types of protective products and supplies for hospital surgical care and ordinary people protection.

PRODUCTION BASE XINFENG,GANZHOU


At the end of January 2020, BQ set up the producion line of Medical coverall and Medical face masks, and started to build the whole industrial chain of spstream raw materials, middle end factory manufacturing and end online and offline sales. To produce high quality personal protrctive producs according to internationalstandards.
CORPORATE R&D CAPABILITIES
LABORATORY


BQ Laboratory has taken the lead in combining new product development with application market research and has supplied High-quality innovative products for the medical, health and civilian markets, It is praised as a research and development vane.
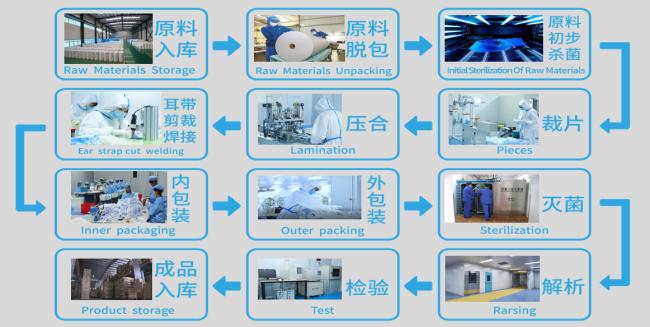
PRODUCTION PROCESS FLOW CHART
QUALITY CONTROL SYSTEM
Strict health standard control




1 . Level 100000 sterile workshop
2 . 10000 level microorganism detection
3 . Biological detection
4 . Production l ine of full automatic
5 . Dust removal in the air shower room
6 . Ethylene oxide sterilization
Technical advantages
The company has established a sound quallity system in accordance with the “medical device production quality management specifications”《SCOPE OF QUALITY MANAGEMENT OF MEDICAL DEVICE PRODUCTION》,and maintained effective operation. The production plant,equipment,human resources and other resources meet the production requirements of products, and the ontrol of procurement, production pocess, inspection and other links is complete and effective.
Process Control
Our company has a 100,000 level sterile medical equipment production workshop of more than 3,000 square meters, equipped with a full-automatic mask production line. More than 100 square meters of 10,000 level microbial testing laboratory is equipped with biosafety cabinet,super clean bench and sterilizing installation.The physical research and development laboratory is more then 100 square meters, equipped with testing and development equipment such as mask filtration performance tester etc. with complete research and development , production and testing capabilities of medical surgical masks and medical protective coverall.
Equipment Test



The comprehensive performance indexes of our company's sterile medical device production workshop and microbiological testing laboratory all meet the requirements of the level control of cleanliness(static) in the clean room(interior) specified in the appendix of “quality management specification for medical device production sterile medical device”.
GLOBAL SUPPLY

The global delivery of BQ series of Medical and protective equipment is available in the United States, France,Germanny,Spain,Peru and other countries. The logistics system is strong, fast and reliable.























